DNA robots get sophisticated by Jef Akst
June 3, 2010 by CLF
Filed under Arthritis, Blog, Gluten-Free Diets/Celiac Disease, Healthy Living, Holistic Nutrition, LEAP Program
Comments Off on DNA robots get sophisticated by Jef Akst
Scientists are one step closer to creating molecular robots that may eventually perform complex tasks, such as building nanomolecules or delivering drugs to target tissues.
They have constructed DNA-based robots that can walk along a specific path unaided or collect various nanoparticles along an assembly line, according to two studies published this week in Nature.
“This has the feel to me of the beginning of a technology revolution,” said Andrew Ellington, an evolutionary engineer at the University of Texas at Austin and the vice president of the International Society for Nanoscale Science, Computation and Engineering, who was not involved in the research. “This work will absolutely pave the way for how you build molecular robots.”
The robots built in one study are a type of DNA walker, called a molecular “spider.” They are minute, mobile molecules that move along a flat surface made out of folded DNA, known as DNA origami, binding to and unbinding from the surface as they go.
The movement of these spiders is largely random, however, said biochemist and study co-author Milan Stojanovic of Columbia University. But together with several other big players in the nanotechnology and DNA computing fields, including Nils Walter of the University of Michigan, Erik Winfree of the California Institute of Technology, and Hao Yan of Arizona State University, Stojanovic designed a DNA origami surface that directed the DNA spider down a specified path (see video).
“You just have to start it, and it walks the path,” said chemist Kurt Gothelf, director of Centre for DNA Nanotechnology at Aarhus University in Denmark, who was not involved in the research.
The spider is fueled by the chemical interactions its single-stranded DNA “legs” have with the origami surface. In order to take a “step,” the legs first cleave a DNA strand on the surface, weakening its interaction with that part of the origami surface. This encourages the spider to move forward, pulled towards the intact surface, where its interactions are stronger. When the spider binds to a part of the surface that it is unable to cleave, it stops.
In essence, the researchers created a DNA spider that can “sense the environment,” Stojanovic said — “molecules that respond [to environmental] cues and behave [in] certain programmable ways on their own.” The next step, he added, is to increase the complexity of movements performed by such autonomous robots by compiling “a collection of rules [of] interactions between molecules and between molecules and environment.”
A fluorescence video microscopy-generated animation of a DNA spider moving along the designated path from the green-labeled start site towards the red-labeled goal. Each colored dot represents its position at a given time over the 40-minute observation period (see legend).
Credit: Nils Walter, Anthony Manzo, Nicole Michelotti and Alexander Johnson-Buck, University of Michigan
Meanwhile, Nadrian Seeman of New York University and his colleagues have designed another type of DNA walker that can collect nano-sized “cargo” as it moves. Unlike the autonomous spider, the cargo-collecting walker is controlled by single strands of DNA added by the researchers to direct the robot. These strands instruct the robot to move past an “assembly line” of three small loading devices, also made out of DNA, each containing a gold nanoparticle. Each loading device can be programmed to either donate its cargo to the passing walker, or keep it, such that the walker can receive anywhere from zero to three particles along its short (less than 200 nanometers) journey.
It’s “like an automobile assembly line,” Seeman said. “We have the option to either add or not add various components to [the walker] depending on how the devices are programmed.”
One possibility for future experiments will be to combine the advances of each of the two papers into one complex, autonomous DNA robot, said Lloyd Smith of the University of Wisconsin, who wrote an accompanying review in Nature. “It’s going to take more work to take it to that next level, [but] bringing those two things together is going to be the next step towards” a fully autonomous, functional nano-sized robot.
Another future direction, the researchers agree, would be to scale up the length of the pathways and the complexity of the behaviors. But even once greater levels of complexity are achieved, what can actually be done with the little robots is still up for debate. “This whole field,” which is still in its early stages, Smith said, “hasn’t really found the application yet.” DNA robots have thus far proven to be capable of fairly sophisticated manipulation at the nanoscale, but the practical uses of this novel technology are still a little unclear.
One popular idea is to use cargo-collecting robots to construct nanomolecules that would be difficult to make using traditional methods, because of the control they offer researchers at such a tiny scale. “The ability to hold a molecule in a particular position and hold another molecule in a defined position could open up possibilities in organic synthesis,” said Smith. Another possibility is their use in drug delivery, said biochemist William Shih of Harvard University, who did not participate in the studies. “Having a very smart robotic delivery system could do a lot better job of killing the disease tissue and do far less damage to our otherwise healthy tissue,” he explained.
But most agree that these potential applications are yet to be realized; the current work merely shows “proof of principle” that such complex behavior might one day be achieved using this technology, Seeman said.
“I think these are both really, really significant papers, not because of what we can do with [these robots] now, but because of what we can do with them in the future,” said Ellington. They are “paving the way to a future where we can do practical DNA technology.”
H. Gu, et al., “A proximity-based programmable DNA nanoscale assembly line,” Nature, 465:202-5, 2010.
K. Lund, et al., “Molecular robots guided by prescriptive landscapes,” Nature, 465:206-10, 2010
Read more: DNA robots get sophisticated – The Scientist – Magazine of the Life Sciences http://www.the-scientist.com/blog/display/57400/#ixzz0oz3tm7it
Celiac Disease
January 26, 2010 by CLF
Filed under Blog, Gluten-Free Diets/Celiac Disease
Disease characteristics. Celiac disease is a systemic immune disease that can be associated with gastrointestinal findings (diarrhea, weight loss, abdominal pain, anorexia, lactose intolerance, abdominal distention, and irritability) and/or highly variable non-gastrointestinal findings (iron-deficiency anemia, dermatitis herpetiformis, chronic fatigue, joint pain/inflammation, migraines, depression, attention-deficit disorder, epilepsy, osteoporosis/osteopenia, infertility and/or recurrent fetal loss, vitamin deficiencies, short stature, failure to thrive, delayed puberty, dental enamel defects, and autoimmune disorders). Classic celiac disease, characterized by mild to severe gastrointestinal symptoms, is less common than nonclassic celiac disease, characterized by absence of gastrointestinal symptoms.
Diagnosis/testing. The diagnosis of celiac disease relies on characteristic histologic findings on small-bowel biopsy and clinical and/or histologic improvement on a gluten-free diet. Most individuals with celiac disease have celiac disease-associated antibodies and specific pairs of allelic variants in two HLA genes, HLA-DQA1 and HLA-DQB1. Because 30% of the general population has one of the celiac disease-associated HLA alleles and only 3% of individuals with one or both of these alleles develop celiac disease, presence of celiac disease-associated HLA alleles is not diagnostic of celiac disease; however, their absence essentially excludes a diagnosis of celiac disease.
Management. Treatment of manifestations: lifelong adherence to a strict gluten-free diet (avoidance of wheat, rye, and barley); treatment of nutritional deficiencies (iron, zinc, calcium, fat-soluble vitamins, folic acid); standard treatment of osteoporosis. Prevention of primary manifestations: lifelong gluten-free diet. Surveillance: for symptomatic individuals responsive to a gluten-free diet, periodic physical examination and assessment of growth, nutritional status, and non-gastrointestinal disease manifestations; repeat small-bowel biopsy one to three years following diagnosis. For symptomatic individuals unresponsive to a gluten-free diet, periodic evaluation for refractory sprue, ulcerative enteritis, T-cell lymphoma, and other gastrointestinal cancers. Agents/circumstances to avoid: dietary gluten. Testing of relatives at risk: when the celiac disease-associated HLA alleles in the family are known, molecular genetic testing of first-degree relatives (including young children) to monitor those with known celiac disease-susceptibility alleles for early evidence of celiac disease in order to institute gluten-free diet early in the disease course.
Genetic counseling. Celiac disease is a multifactorial disorder resulting from the interaction of HLA-DQA1 and HLA-DQB1 gene variants known to be associated with celiac disease susceptibility, less well-recognized variants in non-HLA genes, gliadin (a subcomponent of gluten), and other environmental factors. Some empiric risk data are available for at-risk relatives.
Diagnosis
Clinical Diagnosis
The diagnosis of celiac disease is made through the combination of the following [Hill et al 2005, NIH Consensus Committee 2005, Green & Cellier 2007]:
· Small-bowel biopsy that shows characteristic histologic abnormalities
· Subsequent improvement (clinical and/or histologic) on a gluten-free diet
· Additional findings in most affected individuals:
· Clinical findings or abnormal laboratory findings (although some individuals are asymptomatic and lack laboratory abnormalities)
· Celiac disease-associated antibodies
Note: Although positive specific antibody testing is highly associated with celiac disease and greatly facilitates its diagnosis [Fasano 2001, Farrell et al 2002], small-bowel biopsy remains the gold standard in confirming the diagnosis of celiac disease.
· Celiac disease-associated human leukocyte antigen (HLA) alleles
Testing
Celiac-associated antibody testing
Note:
(1) It is important for the individual being tested to remain on a gluten-containing diet before celiac disease-associated antibody testing and small-bowel biopsy are performed because antibody levels and histologic abnormalities gradually revert to normal on a gluten-free diet.
(2) For individuals on a gluten-free diet, diagnostic celiac disease-associated antibody testing and small-bowel biopsy should follow a gluten challenge (i.e., eating gluten-containing foods [the equivalent of one to three slices of bread per day] for one to three months and sometimes longer if no symptoms are observed). However, the gluten challenge can make some individuals very ill.
· Tissue transglutaminase (tTG) IgA. Measurement of serum concentration of tissue transglutaminase (tTG) immunoglobulin A (IgA) is often recommended for initial testing because of its high sensitivity and specificity for celiac disease, relatively low cost, and ease of test performance and reliability. However, the sensitivity and specificity differ among laboratories [Abrams et al 2006]. False positive test results may occur in persons with acute coronary syndromes and in individuals with cirrhosis and chronic liver disease.
· Endomysial antibody (EMA) IgA. Serum concentration of endomysial antibody (EMA) IgA has the highest specificity (~99%), but is more expensive and more time-consuming to perform and is potentially more prone to false negative results than serum concentration of tTG IgA. Because it is determined by indirect immunofluorescence, serum concentration of EMA IgA is subject to observer variability, which affects its sensitivity [Murray 2004]. When performed in an experienced laboratory, this test has a higher specificity (approaching 100%) than tTG antibody testing and is useful in individuals with cirrhosis.
· Anti-deamidated gliadin-related peptide (a-DGP) antibodies IgA and IgG. This new test detects antibodies binding synthetic deamidated gliadin-related peptides (DGPs). In preliminary studies examining groups with a high prevalence of celiac disease, both isotypes (IgA and IgG) were shown to be highly sensitive and specific for active celiac disease. Specificity is greater than in antigliadin (AGA) testing and similar to that for tTG testing. An increase in DGP antibody levels may precede an increase in serum concentration of tTG-IgA in young children [Liu et al 2007, Niveloni et al 2007]. However, as in all antibody tests, a minority of individuals have false negative results.
· Measurement of serum concentration of total IgA to evaluate for selective IgA deficiency. The prevalence of selective IgA deficiency, a condition of unknown cause, is 1:700 in the general population. For unknown reasons the prevalence of selective IgA deficiency is higher (1:50) in individuals with celiac disease than in the general population [Wong el al 2003, Alaedini & Green 2005].
Note: Because individuals with selective IgA deficiency do not produce IgA antibodies, the celiac-associated IgA antibodies tTG IgA and EMA IgA are not present in these individuals. Therefore, in these individuals, testing for celiac-associated IgG antibodies (tTG IgG) or DGP-IGG should be performed instead.
· Antigliadin antibody (AGA) IgA and IgG. The NIH Consensus Development Conference on Celiac Disease recommended against the use of AGA in the diagnosis of celiac disease because of the low specificity of this assay and the availability of more specific and sensitive tests, including tTG and EMA IgA [Hill et al 2005].
Note: (1) The overall sensitivity of celiac disease-associated antibody testing may be slightly increased when all four tests (serum concentrations of tTG IgA, EMA IgA, total IgA, and AGA IgA and IgG) are performed. However, the use of panels that incorporate AGA markedly increase the false positive rate as a result of a lone positive AGA antibody and drop the positive predictive value to low levels except in the case of a very high pre-test prevalence. (2) Although a positive result on celiac disease-associated antibody testing is likely to be diagnostic of celiac disease, false positive results occur. (3) Conversely, normal celiac-associated antibody test results do not exclude the diagnosis of celiac disease, especially in the presence of lesser degrees of villous atrophy or in persons on a gluten-free diet prior to testing.
Small-bowel biopsy generally refers to multiple (four or more) biopsies taken endoscopically from the post-bulbar duodenum.
Characteristic histologic findings that are the gold standard for the diagnosis of celiac disease include partial or complete villous atrophy, crypt hyperplasia, and increased intraepithelial lymphocytes (IELs). Based on the dynamic development of the pattern of the intestinal lesions and the frequency of mild lesions in celiac disease, Marsh [1992] proposed a four-stage grading classification to establish the diagnosis and to assess improvement in response to a gluten-free diet (Table 1). Although these changes are not unique to celiac disease, reversion of intestinal damage after gluten withdrawal is unique to celiac disease. The positive predictive nature of the biopsies depends on the relative prevalence of celiac disease as compared to other causes of enteropathy in the population.
Authored by:
Cara L Snyder, MS, CGC
Certified Genetic Counselor
Kimball Genetics, Inc
Denver
Danielle O Young, MS, CGC
Certified Genetic Counselor
Kimball Genetics, Inc
Denver
dyoung@kimballgenetics.com
Peter HR Green, MD
Director, Celiac Disease Center
Professor of Clinical Medicine
Columbia University
New York
pg11@columbia.edu
Annette K Taylor, MS, PhD, FACMG
President and CEO
Kimball Genetics, Inc.
Denver
aktaylor@kimballgenetics.com 03072008celiac
Initial Posting: July 3, 2008.
Source: Gene Reviews; funded by the NIH Developed at the University of Washington, Seattle 1993-2009
Alpha-lipoic acid improves vascular endothelial function in patients with type 2 diabetes: a placebo-controlled randomized trial.
January 21, 2010 by CLF
Filed under Blog, Gluten-Free Diets/Celiac Disease
Comments Off on Alpha-lipoic acid improves vascular endothelial function in patients with type 2 diabetes: a placebo-controlled randomized trial.
Heinisch BB, Francesconi M, Mittermayer F, Schaller G, Gouya G, Wolzt M,
Pleiner J.
Medical University of Vienna, Vienna, Austria.
Eur J Clin Invest 2009 Abstract Objective The aim of this study was to investigate the effect of alpha-lipoic acid (ALA) treatment on endothelium-dependent and -independent vasodilatation, assessed by forearm blood flow (FBF), in patients with type 2 diabetes mellitus. Research design and methods A total of 30 subjects with type 2 diabetes were included in this randomized, controlled, double-blinded, parallel group study. FBF responses to intra-arterial acetylcholine (ACh) and glycerol trinitrate (GTN) were measured before and after 21 days of intravenous treatment with 600 mg alpha-lipoic acid or placebo. Results FBF responses were comparable at baseline. After treatment, FBF reactivity to ACh and GTN was unchanged in subjects receiving placebo. By contrast, ALA treatment increased endothelium-dependent vasodilatation to ACh (P < 0.05) but not to GTN compared with baseline. Conclusions Intravenous ALA treatment improves endothelium-dependent vasodilatation in patients with type 2 diabetes, in the absence of effects on forearm vasomotor function. If this salutary action translates into vascular risk reduction remains to be established.
Finding Hidden Gluten in Your Foods
August 25, 2009 by CLF
Filed under Blog, Gluten-Free Diets/Celiac Disease
Following a gluten-free diet can be challenging at the best of times, and successfully avoiding gluten requires a bit of detective work. It may not be obvious when reading a food label that a particular food contains gluten. Below is a list of the many food ingredients and additives that may contain hidden gluten. If you are gluten intolerant, it is important to verify specific foods to ensure that they do not contain hidden gluten.
Baking Powder
Binders
Blue Cheese
Brown rice syrup
Caramel colorings or flavorings
Cereal fillers, protein or starch
Citric Acid
Coatings
Colorings
Corn Starch
Curry Powder
Dextrins
Dispersing Agents
Emulsifiers
Excipients (in prescription medications, for consistency)
Extracts (in grain alcohol)
Fillers
Flavorings (in grain alcohol)
Flours made from wheat, barley, oats, and rye
Grain alcohol (beer, ale, rye, scotch, bourbon, grain vodka)
Gum base
Homeopathic remedies
Hydrolyzed protein, Hydrolyzed plant protein (HPP), Hydrolyzed vegetable protein (HVP)
Malt or Malt Flavoring (barley malt, malt vinegar)
Maltodextrin
Modified starch, Modified food starch (made from wheat)
Mono- and di-glycerides (made using a wheat starch carrier)
Natural flavorings
Oils (wheat germ oil and others with gluten additives)
Preservatives
Soy sauce (when fermented with wheat)
Spices (if anti-caking agent used)
Starch (modified food starch, edible starch)
Textured vegetable protein (TVP)
Vegetable gum
Vegetable protein or starch
Vinegars (white, or malt)
Vital wheat gluten (common in soy products)
Vitamin E oil
Written by Elizabeth Daeninck, MS, RD
Published in November 2008
(HealthCastle.com)
Energy/Snack Bars
August 25, 2009 by CLF
Filed under Blog, Gluten-Free Diets/Celiac Disease
Comments Off on Energy/Snack Bars
(gluten free list included)
- Cliff Bars and mini Cliff Bars
- Cliff Nectar Bars
- Cliff Z Bars (good for kids)
- Gnu Bars (high fiber)
- Honey Bars
- Kashi TLC Bars (good fiber, moderate protein)
- Keri Bars
- Lara Bars
- Luna Bars
- Odwalla Bars
- Perfect 10 Bars (dried fruit and nuts)
- You Bars (you make them yourself www.youbars.com)
- Zing Bars
Gluten-free Bars: Perfect 10, Elev8Me, Hammer Bar, EnvirKids Rice Cereal Bar; Omega Smart Bars, Extend Bar, Bumble Bar.ReNew Life Organic Energy Bar, Think5 Bar, Allerenergy Bar, GoMarco Bar, some Lara bars-gluten free
Wheat-free but not gluten-free: Clif Nectar, Clif Builder’s Bar, Odwalla Bar.
New Horizons for Whole Grains and the Gluten-Free Diet
August 19, 2009 by CLF
Filed under Blog, Gluten-Free Diets/Celiac Disease
Comments Off on New Horizons for Whole Grains and the Gluten-Free Diet
 © Carol Fenster, PhD, author of Gluten-Free Quick & Easy,
© Carol Fenster, PhD, author of Gluten-Free Quick & Easy,
www.glutenfreequickandeasy.com
© Shelley Case, RD, author of Gluten-Free Diet: A Comprehensive Resource Guide
www.glutenfreediet.ca
The National Institutes of Health (NIH) state that 1:100 or 3 million Americans have celiac disease, an autoimmune disorder where gluten inhibits the absorption of nutrients from food. The only treatment is a strict lifelong, gluten-free diet. People who are allergic or intolerant to gluten must also avoid it. Gluten is the general name for specific proteins found in the grains wheat, spelt, kamut, rye, triticale, and barley.
What are Whole Grains?
Gluten-free grains (also called cereals) are the seeds of plants and include brown rice, corn, flax, Montina™ (Indiana ricegrass), millet, oats (pure, uncontaminated), sorghum, teff, and wild rice––as well as the pseudo-grains of amaranth, buckwheat, and quinoa. A grain is “whole” when it is consumed in a form that includes the bran (outer layer and primary source of fiber), germ (the part that sprouts into a new plant), and endosperm (the bulk of the seed).
The Scoop on Oats
Until recently, oats were not allowed in the gluten-free diet because the protein in oats was thought to trigger the same toxic reaction as wheat and other gluten-containing grains. New research in Europe and the US over the past 14 years has revealed that consumption of moderate amounts of oats is safe for the majority of children and adults with celiac disease. Most of these studies used pure, uncontaminated oats, but it should be noted that a very small number of persons with celiac disease may not even tolerate pure oats. The mechanism causing this intolerance has yet to be established.
Based on this new research, a growing number of celiac organizations and health professionals around the world now allow consumption of moderate amounts of pure, uncontaminated oat products in diet. An extensive technical review on the safety of oats was recently published on Health Canada’s website.
Unfortunately, the majority of commercial oats products on the market are cross-contaminated with wheat, barley, or rye which occurs during harvesting, transporting, storing, milling, processing, and packaging. The good news is that there are companies in the US, Canada, and Europe who produce pure, uncontaminated specialty oat products. The North American companies are Bob’s Red Mill, Cream Hill Estates, FarmPure Foods (Only Oats™), Gifts of Nature, and Gluten-Free Oats.
Health Benefits of Whole Grains in a Gluten-Free Diet
People who regularly eat whole grains have a lower risk of obesity, lower cholesterol levels, and a reduced risk of heart disease, stroke, type 2 diabetes, and cancer. The USDA and the Whole Grains Council recommend 3 to 5 servings of whole grains per day. Look for the yellow Whole Grains Stamp. Eating three whole grain food products labeled “100% Whole Grain”––or six products bearing ANY Whole Grain Stamp–– satisfies the need for 3 to 5 servings per day.
- Add cooked buckwheat, oat groats, steel-cut oats, quinoa, sorghum, or wild rice to rice pilaf
- Enrich soups with cooked brown rice, buckwheat, oat groats, quinoa, sorghum, or wild rice
- Boost nutritional content of brownies, cakes, and cookies with 1/4 cup cooked amaranth or teff
- Sprinkle cooked whole grains over mixed green salads
- Toss cooked whole grains with gluten-free pasta
- Cook whole grains in a slow-cooker overnight for a hearty breakfast (see page 5)
- Dress cold cooked whole grains with pesto or a zesty salad dressing for tabbouleh (see page 4)
- Blend cooked oat groats or brown rice with black beans or pinto beans in Southwestern dishes
- Extend hamburger patties or meat loaf with gluten-free rolled oats or cooked brown rice, quinoa, amaranth, or teff
- Replace 1/4 of the cornmeal with teff grains for a cornmeal-teff polenta
- Add cooked amaranth, quinoa, or teff to puddings for interesting texture
- Cook hot cereal for breakfast from Altiplano Gold quinoa cereals, Ancient Harvest quinoa flakes, Bob’s Red Mill Mighty Tasty GF Hot Cereal, or The Birkett Mills’ buckwheat flakes
- Use quinoa flakes or gluten-free cold cereals and granolas to top fruit crisps
- Choose pasta that is made with added rice bran (e.g., Tinkyada) or with quinoa (e.g. Ancient Harvest, NorQuin)
- Choose baking flours such as amaranth, brown rice, buckwheat, Montina™, quinoa, sorghum, teff, or wild rice because they are ground from the whole grain
- Sprinkle ground flax or flax meal on yogurt or hot cereal
- Add cream of buckwheat cereal, ground flax or flax meal , or rice bran to homemade breads
- Enjoy popcorn as a nutritious snack
- Choose whole grain crackers such as Mary’s Gone Crackers
Nutrient Composition of Gluten-Free Whole Grains Compared to Whole Grain Wheat
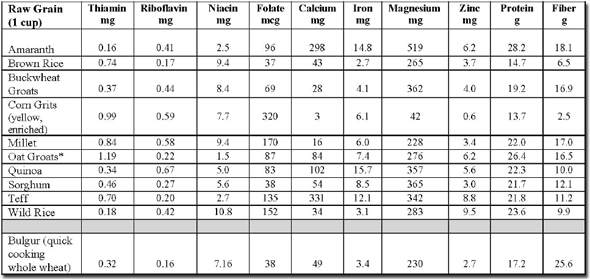
* choose pure, uncontaminated oat groats
Nutrient composition data from:
1. Gluten-Free Diet: A Comprehensive Resource Guide –Revised and Expanded Edition, 2008 by
Shelley Case, RD. www.glutenfreediet.ca (Amaranth, brown rice, buckwheat, millet, quinoa, sorghum, teff
and wild rice)
2. USDA National Nutrient Database for Standard Reference at www.nal.usda.gov/fnic/foodcomp/search/
(Corn grits, oat groats, and bulgur)
How to Cook Whole Grains
From 1000 Gluten-Free Recipes by Carol Fenster, PhD (Wiley, 2008)
(Cooking times may vary by altitude and manufacturer. Season to taste with salt, pepper, or your favorite herbs and spices.)
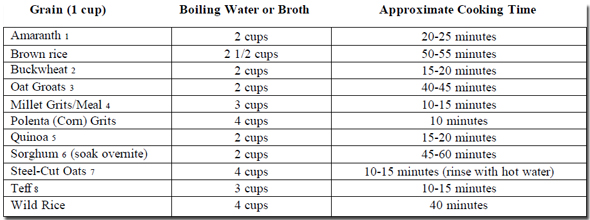
These whole grains are available at health food stores or online from various specialty companies such as:
- www.nuworldamaranth
- www.bobsredmill.com, www.thebirkettmills.com
- www.bobsredmill.com, www.creamhillestates.com, www.onlyoats.com
- www.bobsredmill.com
- www.bobsredmill.com, www.quinoa.com, www.quinoa.net
- www.twinvalleymills.com
- www.bobsredmill.com, www.creamhillestates.com, www.onlyoats.com
- www.bobsredmill.com, www.teffco.com
Quinoa Tabbouleh
(Adapted from 1000 Gluten-Free Recipes by Carol Fenster, PhD, Wiley, 2008)
Most of the quinoa we buy today has already been rinsed to rid it of the bitter saponin coating, particularly if it is from www.quinoa.com, www.quinoa.net or imported through Inca Organics. If you’re not sure about the source, rinse it in a sieve until the water runs clear. Saponin, a natural coating that wards off birds and insects, won’t hurt humans but the quinoa tastes better without it.
To cook quinoa
1 teaspoon canola oil
1 cup uncooked quinoa, rinsed twice
1/2 teaspoon table salt
1 can (14.5 ounces) or 1 3/4 cups gluten-free, low-sodium chicken broth, Swanson’s Natural Goodness
3/4 cup water
Tabbouleh
1/4 cup shelled raw pumpkin seeds
1 English (hothouse) cucumber, unpeeled and finely diced
3 green onions, thinly sliced
1 small red bell pepper, cored, seeded, and finely diced
1 small yellow bell pepper, cored, seeded, and finely diced
1/2 cup chopped fresh parsley
1/2 cup chopped fresh cilantro
1/4 cup chopped fresh mint
1/4 cup crumbled feta cheese (optional)
Dressing and Garnish
3 tablespoons fresh lemon juice
2 tablespoons extra-virgin olive oil
1 tablespoon white wine vinegar or rice vinegar
1/4 teaspoon table salt
1/8 teaspoon white pepper
Fresh mint or parsley sprigs for garnish
1. Heat the oil in a medium saucepan over medium heat and toast the quinoa about 4 minutes, shaking the skillet occasionally, until the seeds are light golden brown.
2. Add the chicken broth and water, reduce the heat to low, and cook 15 to 20 minutes, covered, or until the quinoa is tender. Remove from heat and cool 10 minutes. Drain the quinoa well.
3. Combine the cooked quinoa and remaining tabbouleh ingredients except feta cheese in a large serving bowl.
4. Combine the dressing ingredients (except fresh mint or parsley) in screw-top jar and shake vigorously to blend. Pour over quinoa mixture and toss until all the ingredients are thoroughly coated. Cover the bowl and refrigerate 4 hours. Let stand at room temperature 20 minutes before serving. Toss with the feta cheese just before serving. Garnish with fresh mint or parsley. Serves 6.
Shelley Case’s High-Fiber Hot Cereal
(From Gluten-Free Diet: A Comprehensive Resource Guide by Shelley Case, RD, Case Nutrition Consulting Inc., Revised and Expanded Edition, 2008)
This quick, heart-healthy breakfast is packed with fiber and omega-3 fatty acids. Add a spoonful of
brown sugar, chopped nuts and/or dried apricots or raisins for more flavor and extra nutrients
3 tablespoons flax seed meal (ground flax)*
3 tablespoons Cream of Brown Rice Hot Cereal
1 1/3 cups water
Dash of vanilla
1. Combine the first 3 ingredients in a medium-to-large glass bowl.
2. Cook on high in a microwave for 3 to 4 minutes, or until thick and creamy. Stir in vanilla. Serve with brown sugar, nuts and/or dried fruits
* As flax is very high in fiber, it is important to gradually introduce it in small portions until tolerated. Start with 5 tablespoons hot cereal and 1 to 3 teaspoons of ground flax initially and then gradually work up to 3 tbsp. flax and 3 tbsp. hot cereal.
Variations:
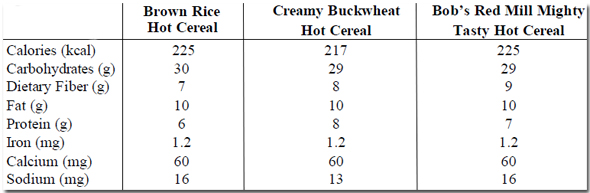
Substitute Creamy Buckwheat Hot Cereal or Bob’s Red Mill Mighty Tasty Gluten-Free Hot Cereal™ for the Brown Rice Hot Cereal.
Nutritional Analysis: 1 serving = 1 cup
Slow Cooker Whole Grain Porridge
(from Gluten-Free Quick & Easy by Carol Fenster, PhD, (Avery, 2007)
Cooked whole grains are an excellent way to start the day, but if you don’t have time in the morning to cook whole grains on the stovetop, use your slow cooker to make porridge overnight. Coat the liner with cooking spray, use a ratio of 1 cup grain to 3 1/2 to 4 cups water or broth, and
cook on Low overnight for 8 to 12 hours. Serve with dried fruit, nuts, and honey, cinnamon, maple syrup, and agave nectar or your favorite sweetener.
Providers of Gluten-Free Whole Grains
www.altiplanogold.com
www.amazinggrains.com
www.arrowheadmills.com
www.bobsredmill.com
www.thebirkettmills.com
www.creamhillestates.com
www.giftsofnature.net
www.glutenfree.com
www.glutenfreemall.com
www.glutenfreeoats.com
www.glutensolutions.com
www.lundberg.com
www.onlyoats.com
www.quinoa.com
www.quinoa.net
www.nuworldamaranth.com
www.teffco.com
www.twinvalleymills.com
For More Information about Using Whole Grains in the Gluten-Free Diet
1000 Gluten-Free Recipes by Carol Fenster, PhD (Wiley 2008)
Gluten-Free Quick & Easy by Carol Fenster, PhD (Avery, 2007)
The Wheat-Free Cook by Jacqueline Mallorca (William Morrow, 2007)
Complete Gluten-Free Cookbook by Donna Washburn and Heather Butt (Robert Rose, 2007)
Gluten-Free Diet: A Comprehensive Resource Guide by Shelley Case, RD (Case Nutrition Consulting Inc., Revised and Expanded Edition, 2008)
Gluten-Free 101 by Carol Fenster, PhD (Savory Palate, 2006)
Cooking Free by Carol Fenster, PhD (Avery, Penguin Group, 2005)
Best Gluten-Free Family Cookbook by Donna Washburn and Heather Butt (Robert Rose, 2005)
Wheat-Free Recipes & Menus by Carol Fenster, PhD (Avery, Penguin Group, 2004)
Food Allergy Survival Guide by Vesanto Melina, MS, RD, Jo Stepaniak, MSEd, Dina Aronson, MS, RD
(Healthy Living Publications, 2004)
Gluten-Free Friends by Nancy Patin Falini, RD (Savory Palate, 2003) -for kids
For More Information on Whole Grains
www.wholegrainscouncil.org
www.mypyramid.gov
www.healthierus.gov/dietaryguidelines

